![The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram](https://www.researchgate.net/publication/309875485/figure/fig1/AS:537898228826112@1505256339484/The-solubility-S-of-dolomite-CaMgCO-3-2-as-a-function-of-pH-LlogH-D.png)
The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram
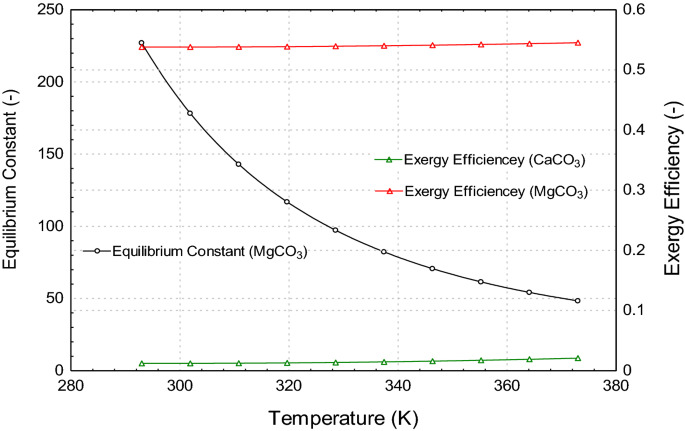
Thermodynamic analysis of theoretical dolomite formation from seawater and captured carbon dioxide | SN Applied Sciences

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

Solubility diagram for the system calcite/Ca-rich dolomite at T = 10°C... | Download Scientific Diagram

Greenhouse conditions induce mineralogical changes and dolomite accumulation in coralline algae on tropical reefs | Nature Communications

Study of Iron-Bearing Dolomite Dissolution at Various Temperatures: Evidence for the Formation of Secondary Nanocrystalline Iron-Rich Phases on the Dolomite Surface | ACS Earth and Space Chemistry
SE - Precipitation of dolomite from seawater on a Carnian coastal plain ( Dolomites, northern Italy): evidence from carbonate petrography and Sr isotopes

Log(K) of calcite-fluid equilibrium (CaCO 3 + H + = HCO 3 − + Ca 2+ )... | Download Scientific Diagram
Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect a
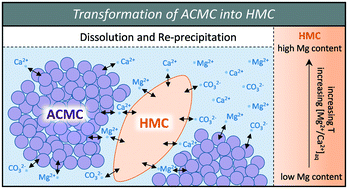
Effect of temperature on the transformation of amorphous calcium magnesium carbonate with near-dolomite stoichiometry into high Mg-calcite - CrystEngComm (RSC Publishing)

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega
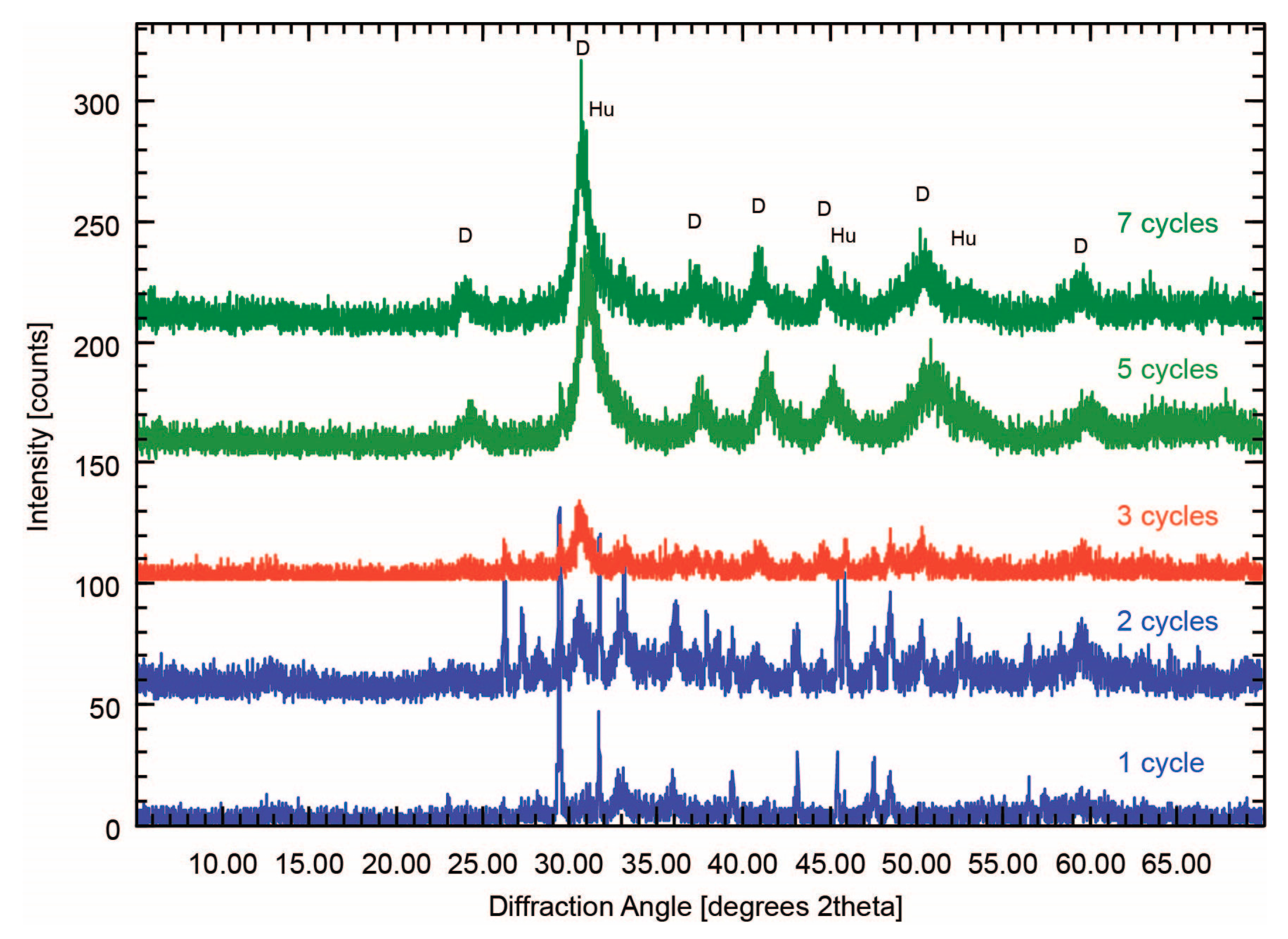
Minerals | Free Full-Text | Effect of pH Cycling and Zinc Ions on Calcium and Magnesium Carbonate Formation in Saline Fluids at Low Temperature

Study of Iron-Bearing Dolomite Dissolution at Various Temperatures: Evidence for the Formation of Secondary Nanocrystalline Iron-Rich Phases on the Dolomite Surface | ACS Earth and Space Chemistry
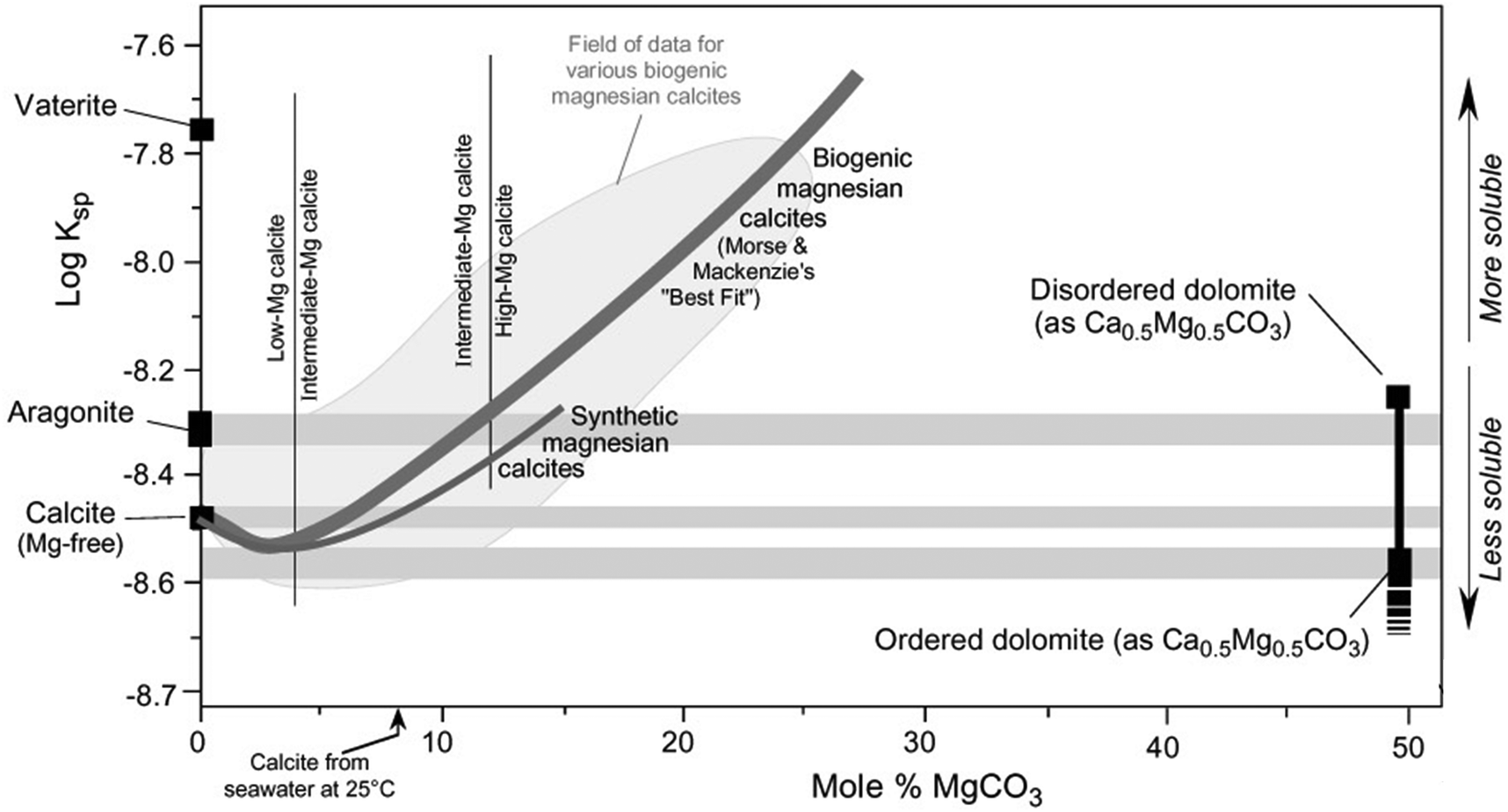
Mechanisms of Mg carbonates precipitation and implications for CO 2 capture and utilization/storage - Inorganic Chemistry Frontiers (RSC Publishing) DOI:10.1039/D2QI02482A
Divalent heavy metals and uranyl cations incorporated in calcite change its dissolution process | Scientific Reports

Solubility and Dissolution Kinetics of Dolomite in Ca–Mg–HCO3/CO3 Solutions at 25°C and 0.1 MPa Carbon Dioxide - Sherman - 2000 - Soil Science Society of America Journal - Wiley Online Library

Calcite solubility in aqueous solution with CO2 in equilibrium obtained... | Download Scientific Diagram








